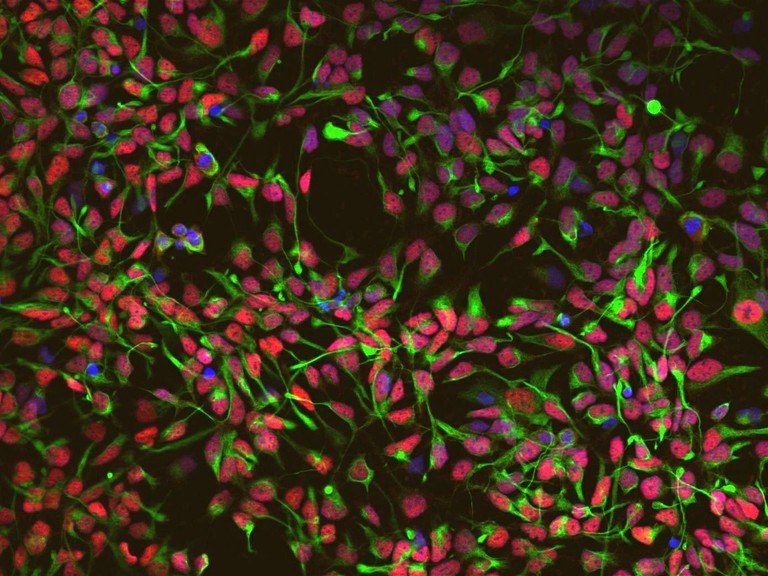
Michael Peitz, manager of the Cell Programming Core Facility, co-directed a study which adressed a key question: Are age-associated epigenetic and transcriptional signatures conserved upon direct conversion of adult human peripheral blood cells (PBCs) into stably proliferating induced neural stem cells (iNSCs) and if to what exent. Employing restricted and integration-free Sendai virus-mediated expression of SOX2 and c-MYC the authors generated a fully functional, bona fide NSC population from PBCs, which remains highly responsive to regional patterning cues and is capable of differentiating into functional neurons and glia. The autors then studied DNA methylation of CpG sites known to reflect age-associated changes of the methylome.
Interestingly, newly converted low passage iNSCs display a profound loss of age-related DNA methylation signatures, which further erodes across extended passaging, thereby approximating the DNA methylation age of isogenic iPSC-derived neural precursors (NPCs). This remarkable epigenetic rejuvenation is accompanied by a lack of age-associated transcriptional signatures and absence of cellular aging hallmarks.